Purdue University News
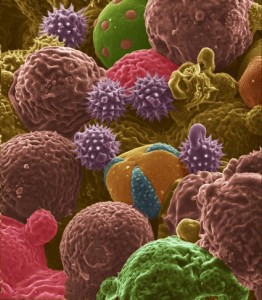
WEST LAFAYETTE, Ind. – Flower pollen could represent a boon for battery makers: Recent research has suggested their potential use as anodes in lithium-ion batteries.
“Our findings have demonstrated that renewable pollens could produce carbon architectures for anode applications in energy storage devices,” said Vilas Pol, an associate professor in the School of Chemical Engineering and the School of Materials Engineering at Purdue University.
Batteries have two electrodes, called an anode and a cathode. The anodes in most of today’s lithium-ion batteries are made of graphite. Lithium ions are contained in a liquid called an electrolyte, and these ions are stored in the anode during recharging.
The researchers tested bee pollen- and cattail pollen-derived carbons as anodes.
“Both are abundantly available,” said Pol, who worked with doctoral student Jialiang Tang. “The bottom line here is we want to learn something from nature that could be useful in creating better batteries with renewable feedstock.”
Research findings are detailed in a paper that appeared Friday (Feb. 5) in Nature’s Scientific Reports.
Whereas bee pollen is a mixture of different pollen types collected by honey bees, the cattail pollens all have the same shape.
“I started looking into pollens when my mom told me she had developed pollen allergy symptoms about two years ago,” Tang said. “I was fascinated by the beauty and diversity of pollen microstructures. But the idea of using them as battery anodes did not really kick in until I started working on battery research and learned more about carbonization of biomass.”
The researchers processed the pollen under high temperatures in a chamber containing argon gas using a procedure called pyrolysis, yielding pure carbon in the original shape of the pollen particles. They were further processed, or “activated,” by heating at lower temperature – about 300 degrees Celsius – in the presence of oxygen, forming pores in the carbon structures to increase their energy-storage capacity.
The research showed the pollen anodes could be charged at various rates. While charging for 10 hours resulted in a full charge, charging them for only one hour resulted in more than half of a full charge, Pol said.
“The theoretical capacity of graphite is 372 milliamp hours per gram, and we achieved 200 milliamp hours after one hour of charging,” he said.
The researchers tested the carbon at 25 degrees Celsius and 50 degrees Celsius to simulate a range of climates.
“This is because the weather-based degradation of batteries is totally different in New Mexico compared to Indiana,” Pol said.
Findings showed the cattail pollens performed better than bee pollen.
The work is ongoing. Whereas the current work studied the pollen in only anodes, future research will include work to study them in a full-cell battery with a commercial cathode.
“We are just introducing the fascinating concept here,” Pol said. “Further work is needed to determine how practical it might be.”
Electron microscopy studies were performed at the Birck Nanotechnology Center in Purdue’s Discovery Park.
The work was supported by Purdue’s School of Chemical Engineering. The electron microscopy studies at Birck were funded by a Kirk exploratory research grant and were conducted by doctoral students Arthur D. Dysart and Vinodkumar Etacheri. An XPS measurement was conducted by Dmitry Zemlyanov at Birck. Other support came from the Hoosier Heavy Hybrid Center of Excellence (H3CoE) fellowship, funded by U.S. Department of Energy.